Mechanism of action
Zegluxen® is a chemically synthesised glucagon-like peptide-1 receptor agonist (GLP-1 RA)1

Zegluxen® is a chemically synthesised glucagon-like peptide-1 receptor agonist (GLP-1 RA)1
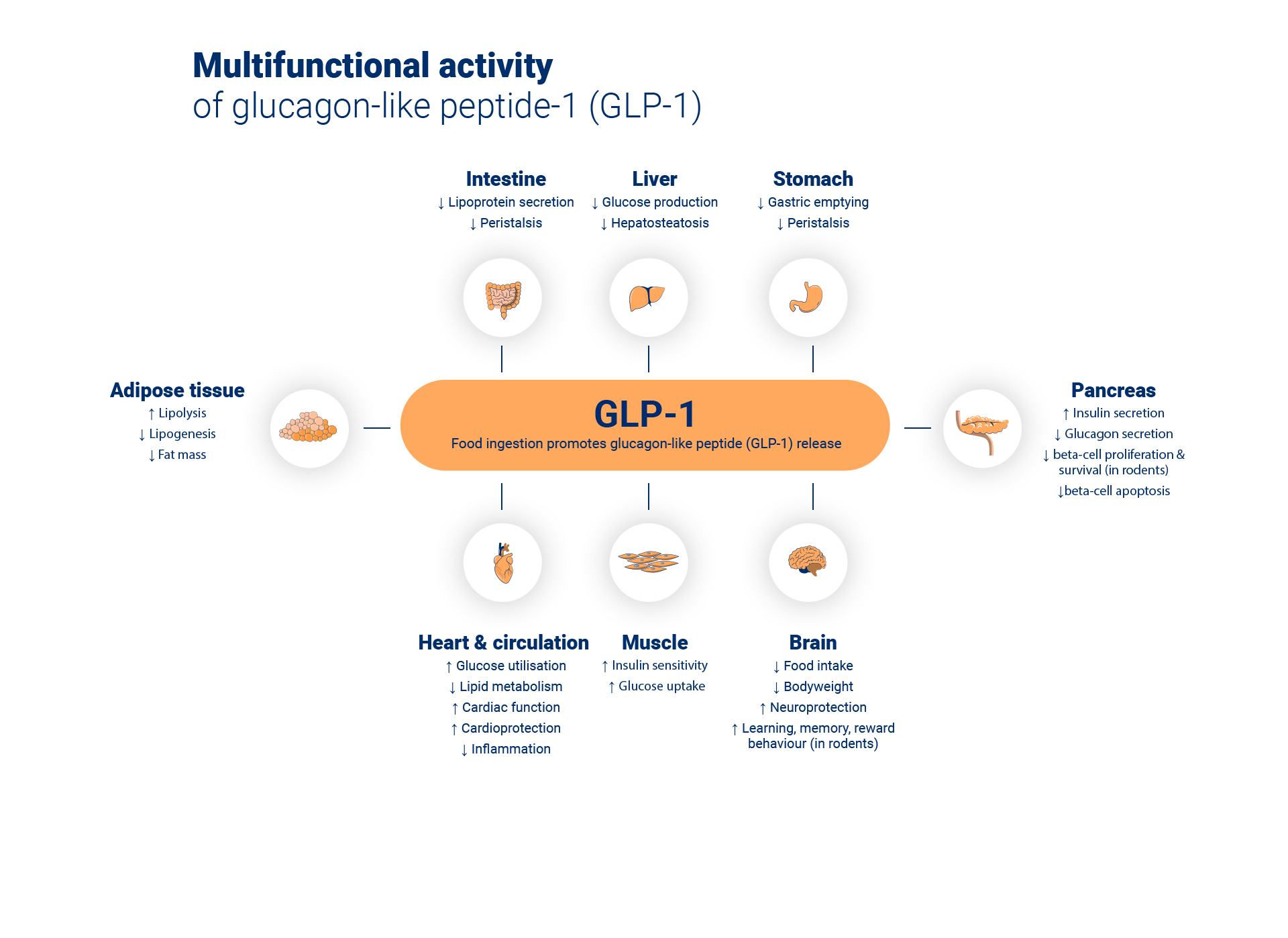
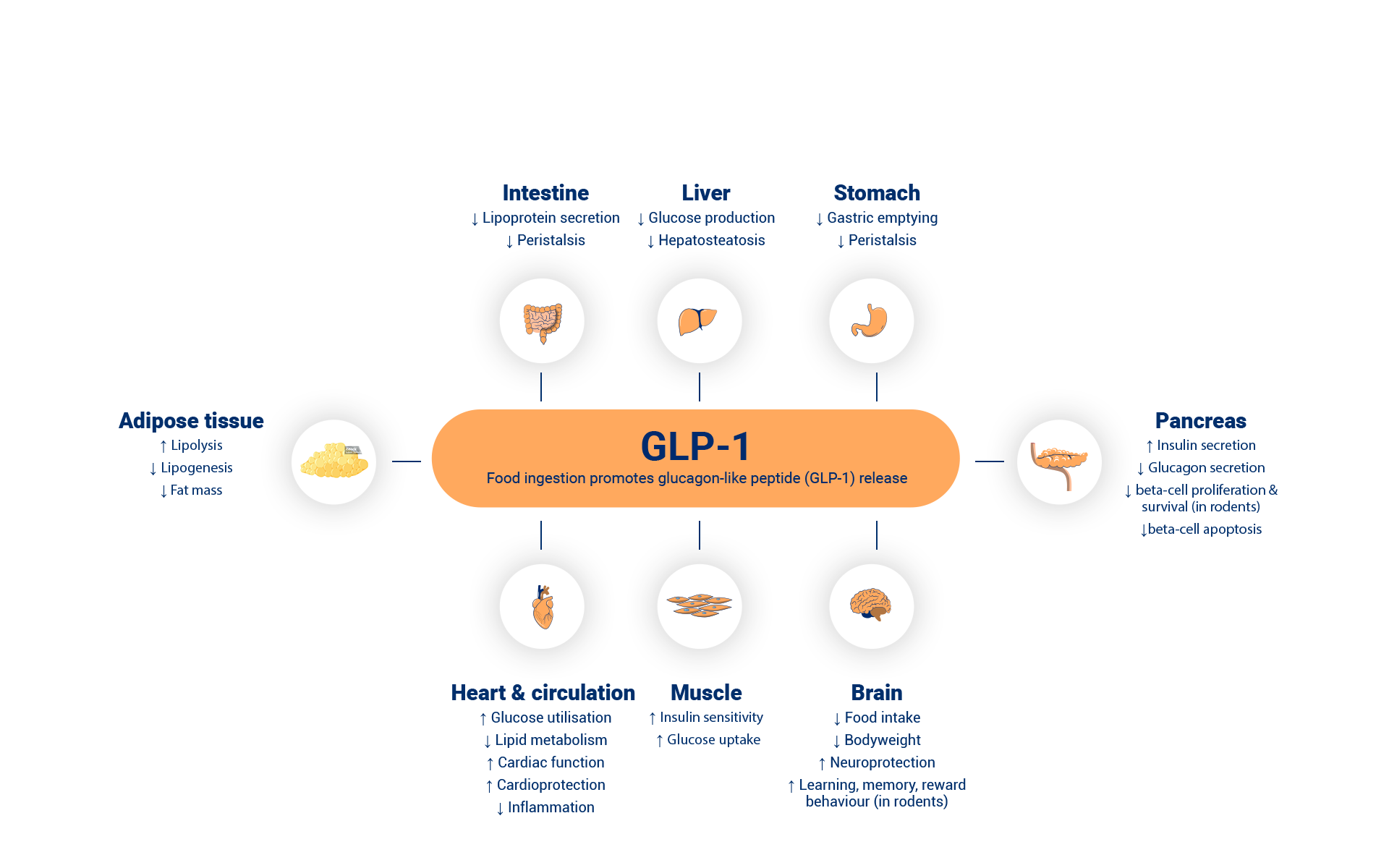
Zegluxen® is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) – an analogue with 97% sequence homology to human GLP-1 peptide that binds to and activates the GLP-1 receptor. The GLP-1 receptor is the target for native GLP-1, an endogenous incretin hormone, which amplifies glucose-dependent insulin secretion from the pancreatic beta cells.1
Zegluxen® possesses a protracted action profile based on three mechanisms: self-association, which results in slow absorption; binding to albumin; and higher enzymatic stability towards the dipeptidyl peptidase-4 (DPP-4) and neutral endopeptidase (NEP) enzymes, resulting in a long plasma half-life.1

What does this mean for your patients?
Improved glycaemic control¹
Liraglutide action is mediated via a specific interaction with GLP-1 receptors, leading to an increase in cyclic adenosine monophosphate (cAMP). When blood glucose is high, insulin secretion is stimulated and glucagon secretion is inhibited by liraglutide, both in a glucose-dependent manner. Conversely, during hypoglycaemia liraglutide diminishes insulin secretion and does not impair glucagon secretion.1
Delayed gastric emptying, reduced appetite and body weight¹
The mechanism of blood glucose lowering also involves a minor delay in gastric emptying. Liraglutide reduces body weight and body fat mass through the physiological regulation of appetite and food intake by GLP-1. The exact mechanism of action is not entirely clear, although animal studies have shown that liraglutide specifically activates the GLP-1 receptor to increase satiety and decrease hunger signals, leading to lower body weight. GLP-1 receptors are also expressed in specific locations in the heart, vasculature, immune system, and kidneys. Liraglutide prevented aortic plaque progression and reduced plaque inflammation in murine models of atherosclerosis as well as demonstrating a beneficial effect on plasma lipids.1
PK/PD profile enables once-daily administration¹
Unlike native GLP-1, liraglutide has a pharmacokinetic and pharmacodynamic profile in humans suitable for once daily administration. The 24-hour duration of action improves glycaemic control in your patients by lowering fasting and postprandial blood glucose in patients with Type 2 diabetes.1
Prolonged action profile¹
Following subcutaneous administration of liraglutide the protracted action profile is based on three mechanisms: self-association, which results in slow absorption; binding to albumin; higher enzymatic stability towards the dipeptidyl peptidase-4 (DPP-4) and neutral endopeptidase (NEP) enzymes, resulting in a long plasma half-life.1
cAMP, cyclic adenosine monophosphate; DPP-4, dipeptidyl peptidase-4; GLP-1 RA, glucagon-like peptide-1 receptor agonist; NEP, neutral endopeptidase; PD, pharmacodynamics; PK, pharmacokinetics..
1. Zegluxen® (liraglutide) Summary of Product Characteristics.
2. Tan Q, Akindehin SE, Orsso CE, Waldner, RC, DiMarchi RD, Müller TD, & Haqq AM (2022). Recent Advances in Incretin-Based Pharmacotherapies for the Treatment of Obesity and Diabetes. Frontiers in Endocrinology, 13, 838410. https://doi.org/10.3389/fendo.2022.838410
000697552 | December 2024