Liraglutide efficacy: Clinical trials
The efficacy and safety of liraglutide for weight management in conjunction with a reduced calorie intake and increased physical activity were studied in the SCALE trials with Saxenda® (liraglutide) of which Nevolat® is a generic; four phase 3 randomised, double-blind, placebo-controlled trials which included a total of 5,358 adult patients.

The efficacy and safety of liraglutide for weight management in conjunction with a reduced calorie intake and increased physical activity were studied in the SCALE trials with Saxenda® (liraglutide) of which Nevolat® is a generic; four phase 3 randomised, double-blind, placebo-controlled trials which included a total of 5,358 adult patients.
Superior weight loss was achieved with liraglutide compared to placebo in obese/overweight patients in all groups studied. Across the trial populations, greater proportions of the patients achieved ≥5% and >10% weight loss with liraglutide than with placebo.1
Specific data on weight loss, responders, time course and cumulative distribution of weight change (%) for trials 1–4 are presented in tables 4 to 8 and figures 1, 2 and 3 of the SmPC.1
Obesity and Prediabetes
Trial 1 (SCALE Obesity & Pre-Diabetes – 1839)1,2
A total of 3,731 patients with obesity (BMI ≥30 kg/m²) or who were overweight (BMI ≥27 kg/m²) with dyslipidaemia and/or hypertension were stratified according to prediabetes status at screening and BMI at baseline (≥30 kg/m² or <30 kg/m²). Patients were randomly assigned patients in a 2:1 ratio to receive once-daily subcutaneous injections of liraglutide at a dose of 3.0 mg (2,487 patients) or placebo (1,244 patients). All 3,731 patients were randomised to 56 weeks of treatment and (2,590 completers) the 2,254 patients with prediabetes at screening were randomised to 160 weeks of treatment.1 The coprimary endpoints were the change in body weight and the proportions of patients losing at least 5% and more than 10% of their initial body weight. Both treatment periods were followed by a 12-week off drug/placebo observational follow-up period.1 Lifestyle intervention in the form of an energy-restricted diet and exercise counselling was background therapy for all patients.1,2
Results
At baseline, the mean (±SD) age of the patients was 45.1±12.0 years, the mean weight was 106.2±21.4 kg, and the mean BMI was 38.3±6.4; a total of 78.5% of the patients were women and 61.2% had prediabetes.2
Mean weight loss at 56 weeks:2

A difference of -5.6 kg; 95% confidence interval, -6.0 to -5.1; P<0.001, with last-observation-carried-forward imputation.
Percentage of patients losing at least 5% their body weight: (P<0.001)2
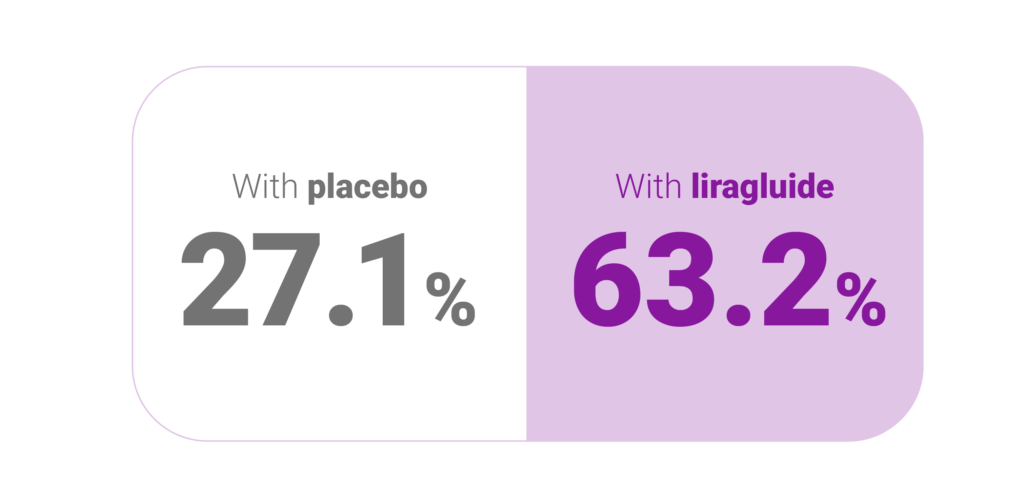
Percentage of patients losing at least 10% their body weight: (P<0.001)2
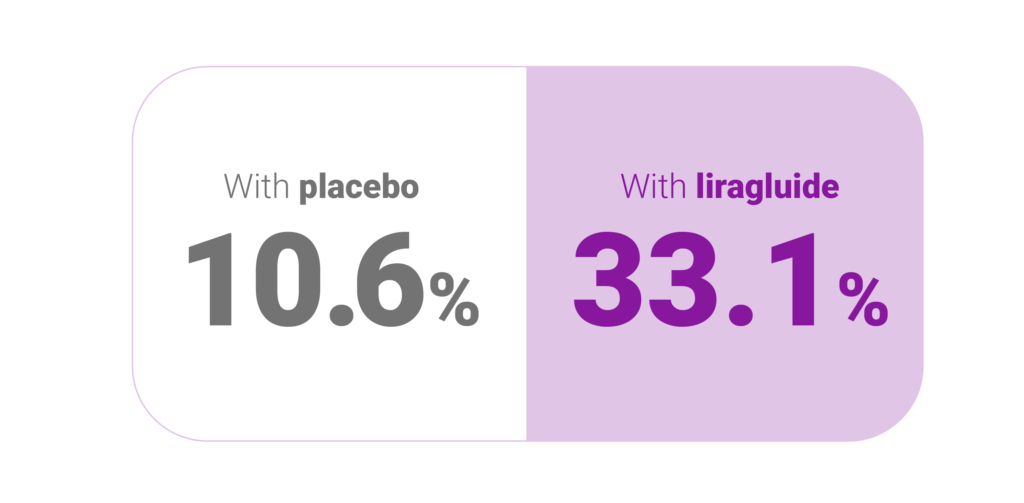
The most frequently reported adverse events with liraglutide were mild or moderate nausea and diarrhoea. Serious events occurred in 6.2% of the patients in the liraglutide group and in 5.0% of the patients in the placebo group.2
Superior weight loss was achieved with liraglutide compared to placebo in obese/overweight patients in all groups studied. As shown, across the trial populations, greater proportions of the patients achieved ≥5% and >10% weight loss with liraglutide than with placebo (please refer to tables 4 to 6 of the SmPC for full data). In the 160-weeks part of trial 1, the weight loss occurred mainly in the first year and was sustained throughout 160 weeks.1,2
Weight loss response after 12 weeks with liraglutide (3.0 mg) treatment1
Early responders were defined as patients who achieved ≥5% weight loss after 12 weeks on treatment dose of liraglutide (4 weeks of dose escalation and 12 weeks on treatment dose). In the 56-week part of trial 1, 67.5% achieved ≥5% weight loss after 12 weeks.1

Diabetes
Trial 2 (SCALE Diabetes – 1922)1,3
A 56-week randomised (2:1:1) trial assessing body weight loss in 846 randomised (628 completers) obese and overweight patients with insufficiently controlled type 2 diabetes mellitus. Inclusion criteria were body mass index of 27.0 or greater, age 18 years or older, taking 0 to 3 oral hypoglycaemic agents (metformin, thiazolidinedione, sulfonylurea) with stable body weight, and glycated haemoglobin level 7.0% to 10.0%.1,3 Patients received once-daily, subcutaneous liraglutide (3.0 mg) (n = 423), liraglutide (1.8 mg) (n = 211), or placebo (n = 212), all as adjunct to 500 kcal/d dietary deficit and increased physical activity (≥150 min/wk). There were three coprimary end points: relative change in weight, proportion of participants losing 5% or more, or more than 10%, of baseline weight at week 56.3
| Parameter measured | Liraglutide
3.0 mg dose |
Liraglutide
1.8 mg dose |
Placebo |
| Baseline weight | 105.7kg | 105.8 kg | 106.5 kg |
| Weight loss % (kg)* | 6.0% (6.4 kg) | 4.7% (5.0 kg) | 2.0% (2.2 kg) |
| Percentage of patients achieving weight loss of ≥5%† | 54.3% | 40.4% | 21.4% |
| Percentage of patients achieving weight loss of ≥10% ‡ | 25.2% | 15.9% | 6.7% |
Results
Among overweight and obese participants with type 2 diabetes, use of subcutaneous liraglutide (3.0 mg) daily, compared with placebo, resulted in weight loss over 56 weeks.
* Estimated difference for liraglutide [3.0 mg] vs placebo, −4.00% [95% CI, −5.10% to −2.90%]; liraglutide [1.8 mg] vs placebo, −2.71% [95% CI, −4.00% to −1.42%]; P < .001 for both).
† Estimated difference for liraglutide [3.0 mg] vs placebo, 32.9% [95% CI, 24.6% to 41.2%]; for liraglutide [1.8 mg] vs placebo, 19.0% [95% CI, 9.1% to 28.8%]; P < .001 for both).
‡ (estimated difference for liraglutide [3.0 mg] vs placebo, 18.5% [95% CI, 12.7% to 24.4%], P < .001; for liraglutide [1.8 mg] vs placebo, 9.3% [95% CI, 2.7% to 15.8%], P = .006).
More gastrointestinal disorders were reported with liraglutide (3.0 mg) vs liraglutide (1.8 mg) and placebo. No pancreatitis was reported.
Sleep Apnoea
Trial 3 (SCALE Sleep Apnoea – 3970)1,4
Obesity is associated with a higher prevalence of obstructive sleep apnoea (OSA) and weight loss has been shown to reduce severity of the disease. SCALE 3 was a 32-week trial assessing whether liraglutide (n=180) could reduce sleep apnoea severity and body weight loss compared with placebo(n=179) in 359 randomised (276 completers) obese patients with moderate or severe OSA. Apnoea severity was measured through changes in apnoea-hypopnoea index (AHI) with an AHI of 15–29.9 events h/hour denoting moderate severity and an AHI ≥ 30 events/hour denoting severe.
Results
Mean reduction in AHI after 32 weeks1,4
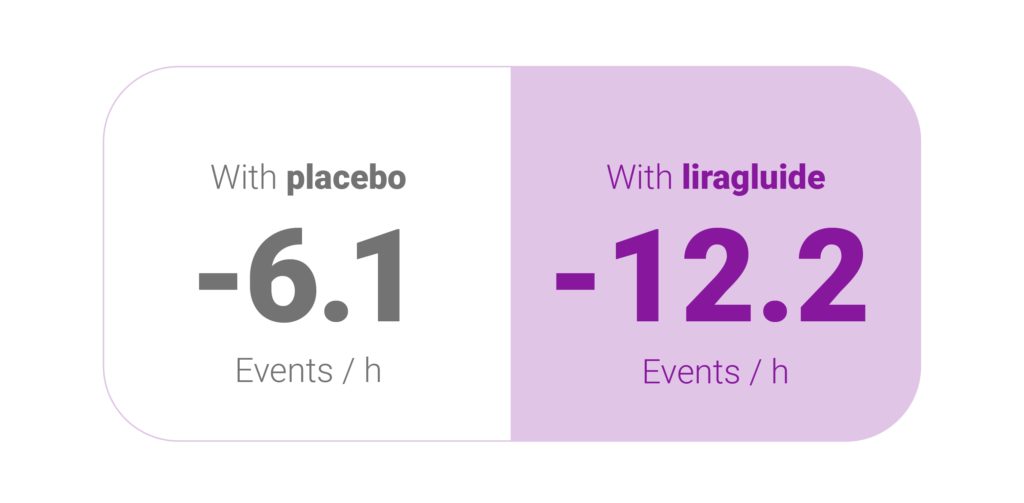
As an adjunct to diet and exercise, liraglutide 3.0 mg was generally well tolerated and produced significantly greater reductions than placebo in AHI, body weight, SBP and HbA1c in participants with obesity and moderate/severe OSA.4
Estimated treatment difference: −6.1 events h− 1
(95% confidence interval (CI), −11.0 to − 1.2), P = 0.0150).

Maintenance
Trial 4 (SCALE Maintenance – 1923)1,5
A 56-week trial assessing body weight maintenance and weight loss in 422 randomised (305 completers, liraglutide 3.0 mg per day or placebo) obese and overweight participants (≥ 18 years, body mass index ≥30 kg m−2 or ≥ 27 kg m−2 with comorbidities) after a preceding weight loss of ≥5% induced by a low-calorie diet (LCD).1,5 Participants received diet and exercise counselling throughout the trial. Co-primary end points were percentage weight change from randomization, the proportion of participants that maintained the initial ≥5% weight loss, and the proportion that lost ≥5% of randomization weight (intention-to-treat analysis).5
Results1,5
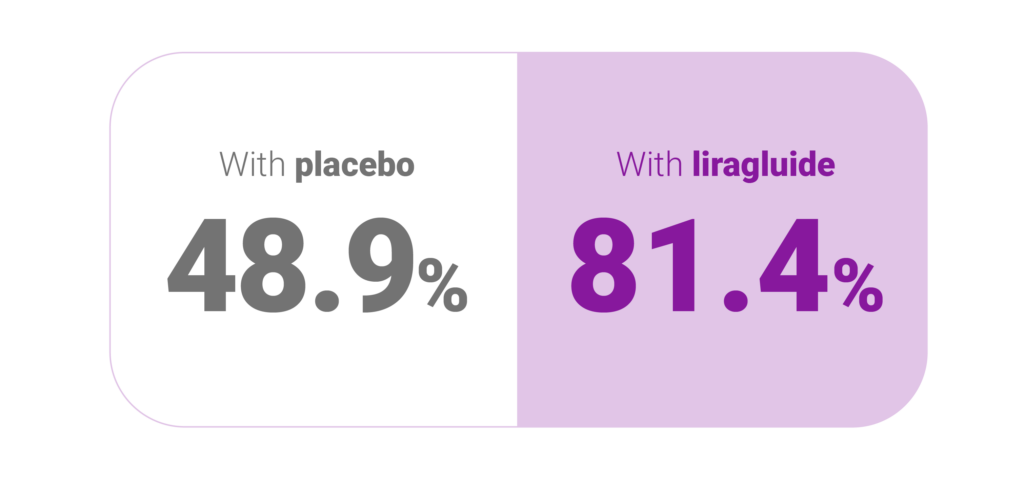
Estimated odds ratio 4.8 (3.0; 7.7), P<0.0001
Percentage of participants who maintained weight loss achieved prior to treatment initiation1,5
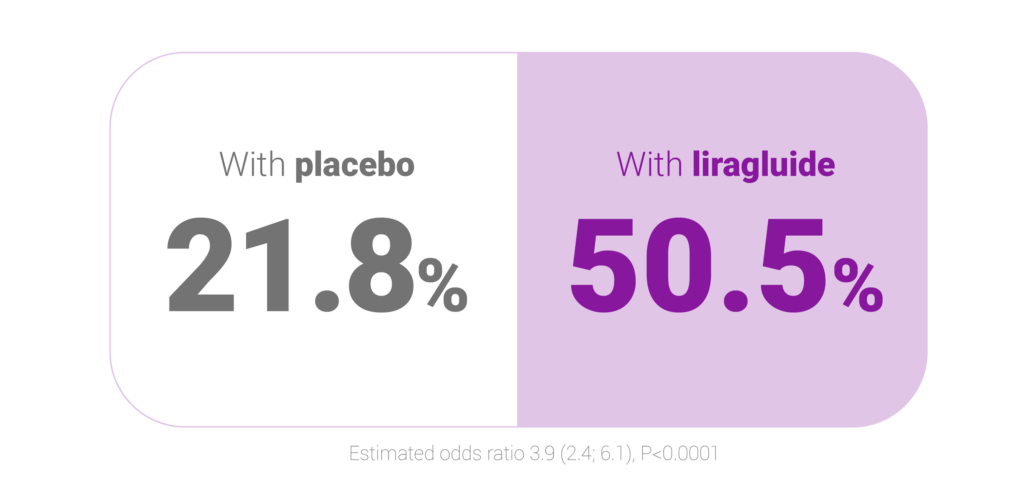
Estimated odds ratio 3.9 (2.4; 6.1), P<0.0001
Percentage of participants who lost ≥5% of randomisation weight5
Liraglutide produced small but statistically significant improvements in several cardiometabolic risk factors compared with placebo. Gastrointestinal (GI) disorders were reported more frequently with liraglutide than placebo, but most events were transient and mild or moderate in severity.
Bioequivalence
Liraglutide bioequivalence was assessed via a randomised, balanced, open-label, two-treatment, two-period, two-sequence, single-dose, crossover bioequivalence, safety and tolerability trial conducted under fasting conditions.6 A single 0.6 mg subcutaneous dose of liraglutide 6 mg/mL solution for injection in a pre-filled pen was assessed as the test product, with Saxenda® as the reference product.
The study included a pilot phase with 6 healthy adult males (5 completed) and a pivotal phase with 24 healthy adult males (all completed). Each subject received either the test or reference drug according to a randomisation schedule, with a 7-day washout period between doses. Serum concentrations of liraglutide were measured using a validated LC MS/MS method. The study lasted 12 days, with a 21-day screening period prior to dosing. Both phases included subjects aged 18-45 years, with a BMI of 18.50-30.00 kg/m² for the pilot phase and 18.50-24.99 kg/m² for the pivotal phase, and minimum weights of 50 kg for males and 45 kg for females.6
Results
Compared with Saxenda® (liraglutide, 6 mg/ml) as the reference product, Nevolat® (liraglutide, 6 mg/ml) met the bioequivalence criteria in terms of rate and extent of absorption after administration of a single dose (both pre-filled pens) under fasting conditions.
Table 1: Pharmacokinetic Parameters of liraglutide test and reference formulations under fasting conditions (N=5)6
(SYNCD-24-22) pilot study
| Mean ± SD of liraglutide | ||
| Parameters (Units) | Test (T) | Reference (R) |
| Cmax (ng/ml) | 27.107 ± 4.9506 | 29.380 ± 2.8494 |
| AUC0-t (ng.hr/ml) | 664.020 ± 112.0304 | 726.066 ± 92.0973 |
| AUC 0-inf (ng.hr/ml) | 714.250 ± 110.9486 | 780.626 ± 114.5194 |
| *Tmax (hr) | 12.50 (10,00, 12.50) | 12.00 (12.00, 16.00) |
| Kel | 0.065 ± 0.0108 | 0.068 ± 0.0054 |
| T1/2 | 10.927 ± 2.2349 | 10.239 ± 0.8547 |
| AUC_Extrapolated | 7.17 ± 2.869 | 6.77 ± 2.207 |
| V2/F (ml) | 13694.620 ± 4501.3143 | 11460.336 ± 1071.0841 |
| CL/F (ml/hr) | 855.857 ± 127.7293 | 781.647 ± 111.7651 |
Table 2: Summary of Bioequivalence Study Results Based on Plasma liraglutide Concentrations
Pooled Data for SYNCD-024-22
| Pharmacokinetic parameter | Geometric Least Squares Mean Test (Test, T) | Least Squares Means (Ref, R) | Ratio (T/R) (%) | 90% Confidence Limits (T vs R) | Intra Subject CV % | Power % |
| Ln AUC0-inf
(ng.hr/ml) |
691.0695 | 757.2532 | 91.26 | (81.82, 101.78) | 7.19 | 95.83 |
| Ln AUC0-t
(ng.hr/ml) |
639.5438 | 707.8203 | 90.35 | (82.14, 99.38) | 6.28 | 97.67 |
| Ln (Cmax) (ng/ml) | 26.0545 | 28.8949 | 90.17 | (82.03, 99.11) | 6.23 | 97.74 |
*Median (Minimum, Maximum) values reported for Tmax; SD – Standard Deviation;
(T) Test, a single dose of liraglutide 6 mg/ml solution for injection in pre-filled pen under fasting conditions
(R) Reference; a single dose of Saxenda® 6 mg/ml solution for injection in pre-filled pen under fasting conditions
AHI, apnoea-hypopnoea index; BMI, Body Mass Index; HbA1c, haemoglobin A1c; GI, gastrointestinal; LCD, low calorie diet; OSA, obstructive sleep apnoea;
P, probability; SBP, systolic blood pressure; SD, standard deviation; SmPC, Summary of Product Characteristics.
- Nevolat® (liraglutide) Summary of Product Characteristics.
- Pi-Sunyer X, Astrup A, Fujioka K et al. A Randomised, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. New Engl J Med 2015;373:11–22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients with Type 2 Diabetes. JAMA 2015;314(7):687–699
- Blackman A, Foster GD, Zammit G, et al. Effect of Liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea Randomized clinical trial. Intl J Obes 2016;40(8):1310–1319
- Wadden TA, Hollander P, Klein S, et al. Weight Maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Intl J Obes (2013) 37(11):1443–1551
- Liraglutide bioequivalence. Data on File. Zentiva, August 2024
000697585 | December 2024